Productos
Nuestras marcas y soluciones terapéuticas han liderado la industria del cuidado de la continencia durante más de 40 años.
Nuestras marcas y soluciones terapéuticas han liderado la industria del cuidado de la continencia durante más de 40 años.

Wellspect desarrolla y comercializa sondas urinarias para cateterismo intermitente desde hace más de 40 años, con nuestra exclusiva tecnología de superficie Urotonic (UST) en equilibrio con el cuerpo para asegurar un sondaje suave y seguro.
Las sondas Wellspect son sondas hidrofílicas intermitentes, fáciles de usar, en equilibrio con tu cuerpo, que te proporcionan comodidad y seguridad a largo plazo.

El contenido de esta sección está restringido solo a Profesionales de la Salud, tal y como recoge el Real Decreto 9/1996 de 15 de enero en relación a los productos sanitarios reembolsados por el Sistema Nacional de Salud. Para acceder a la información completa, deberá darse de alta como Profesional de la Salud.

Navina Systems son nuestras soluciones de irrigación transanal de alto volumen (ITA). Wellspect ha desarrollado sistemas de ITA de control manual y electrónico para aliviar eficazmente la incontinencia fecal y/o el estreñimiento crónico.
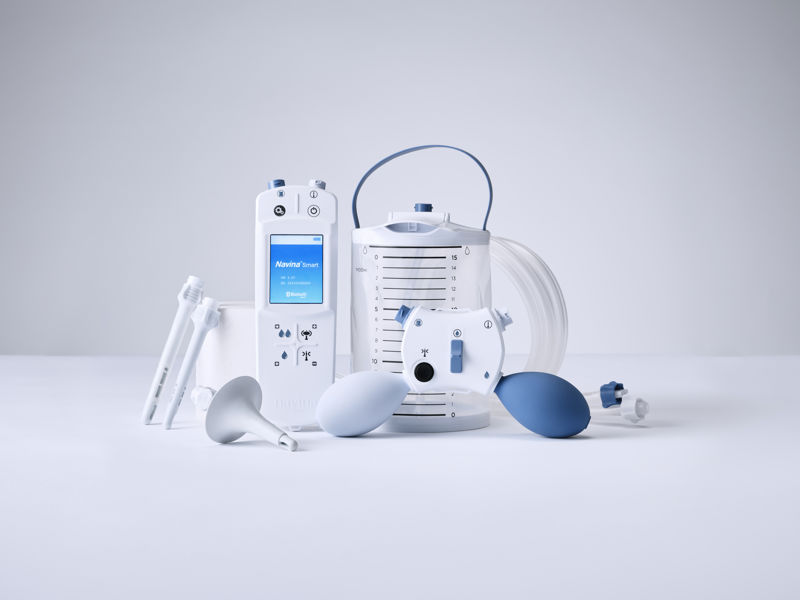
key:global.content-type: Productos
Navina Insert es un obturador anal suave y flexible diseñado para prevenir la fuga involuntaria de heces. Se ha desarrollado para proporcionar un doble sellado y evitar así las pérdidas. Gracias a su forma, se adapta al cuerpo y ofrece un confort único a los usuarios.
Pide información